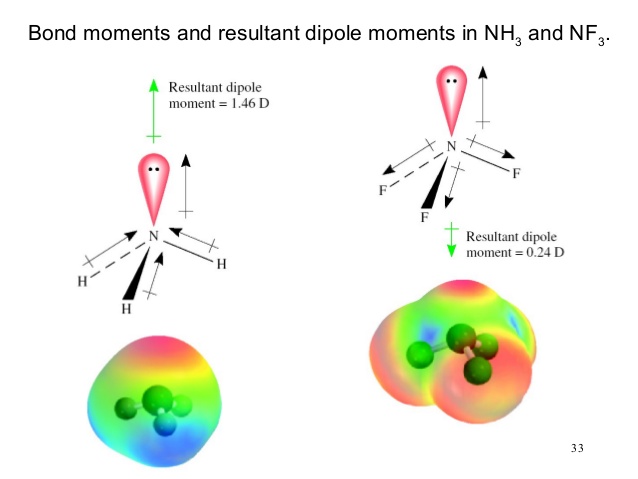
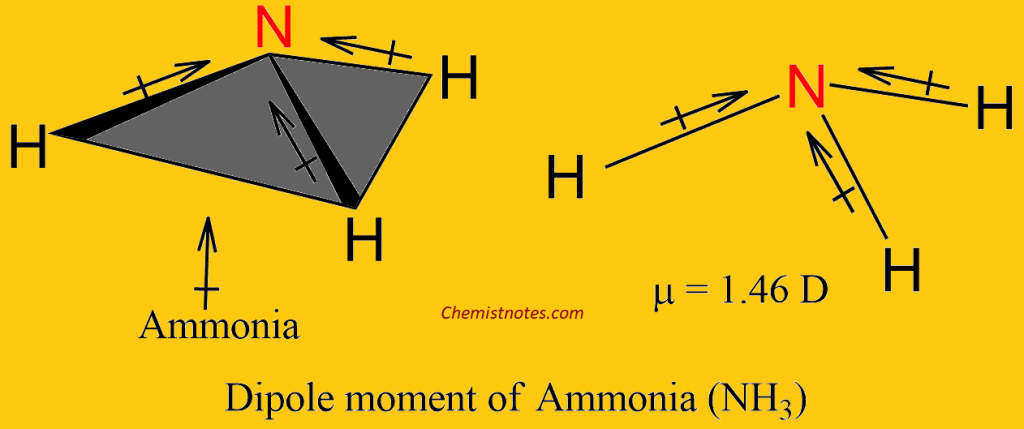
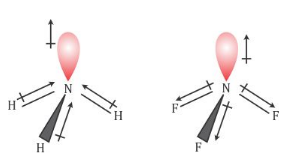
17. The electronegativity difference between N F is greater than that between N H. Yet the dipole moment of NH3 is 1.5D. This is because: 1. In NH3 the atomic dipole and
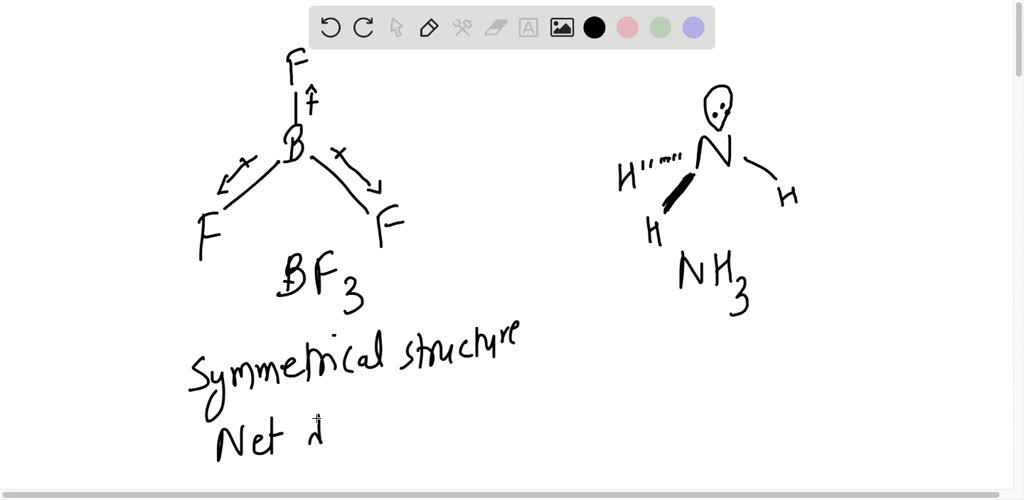
Dipole moment of NF3 is less than that of NH3, even though N-F bond is more polar than N-H bond. Explain. - Sarthaks eConnect | Largest Online Education Community

Effect of on dipole moments of CO, O3, NH3 molecules Dipole moment |P e... | Download Scientific Diagram






![Solved D) Has larger dipole moment (NH3, Or NF3] NH3 has | Chegg.com Solved D) Has larger dipole moment (NH3, Or NF3] NH3 has | Chegg.com](https://media.cheggcdn.com/study/a4f/a4f8fe7f-ba32-4d59-a27f-a1f5fd47c576/image.png)



![SOLVED] The correct order of dipole moment is CH4<NF3<NH3<H2O - Self Study 365 SOLVED] The correct order of dipole moment is CH4<NF3<NH3<H2O - Self Study 365](https://static.tllms.com/moodle-migration/15617_9a10c2f086a16e947dad40a0c7d39c2ec62a11be_nhna.png)