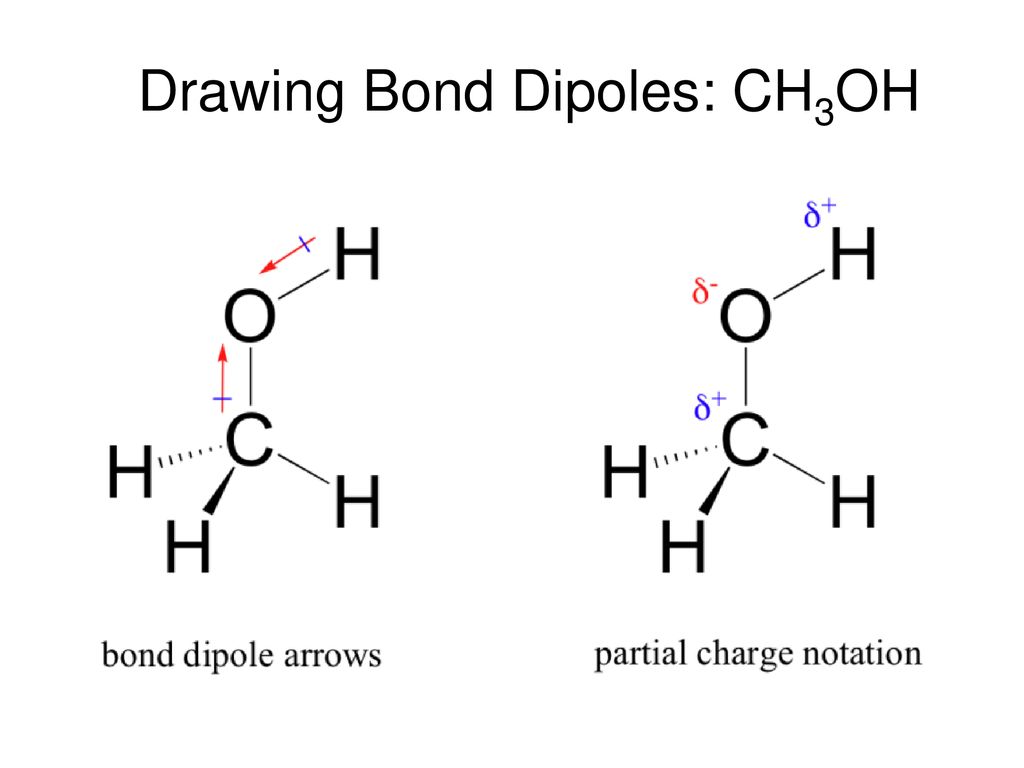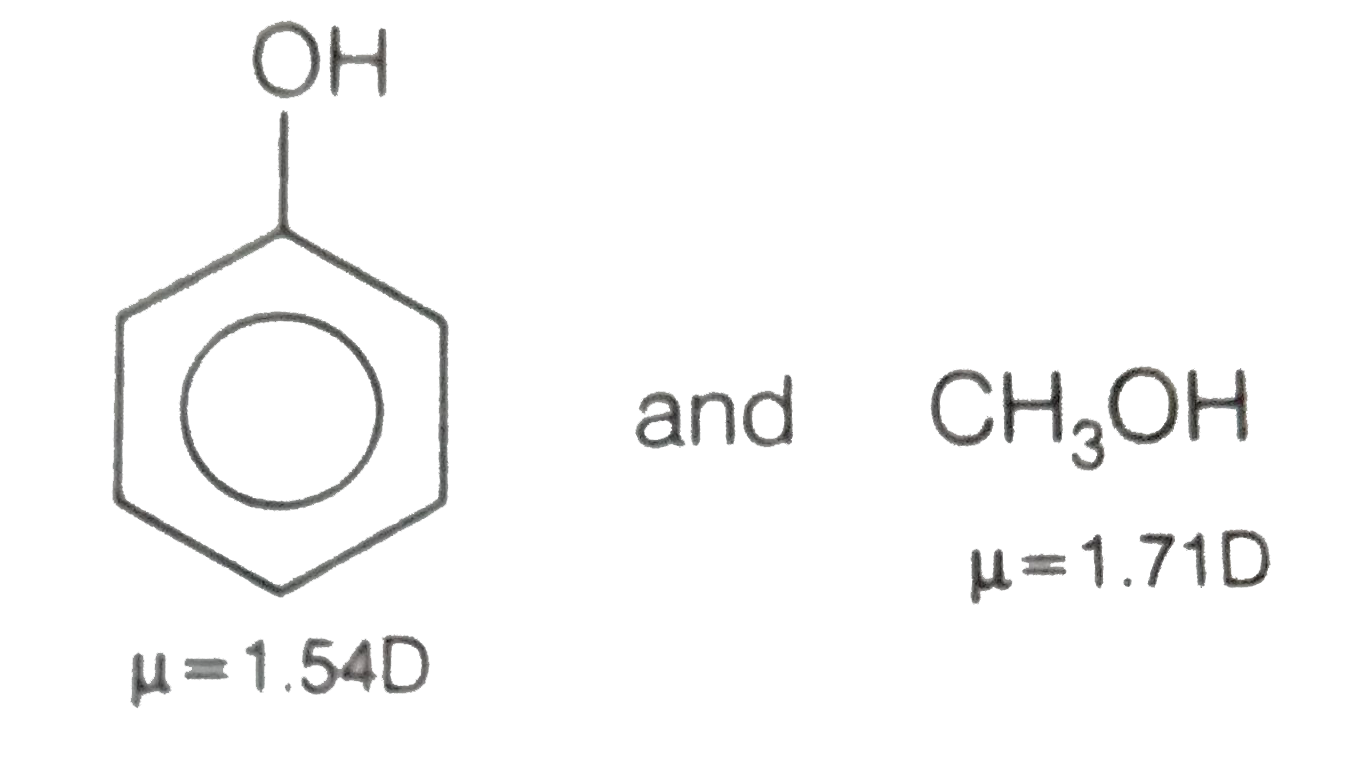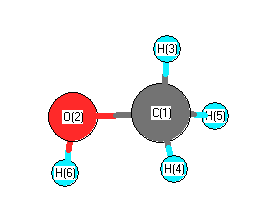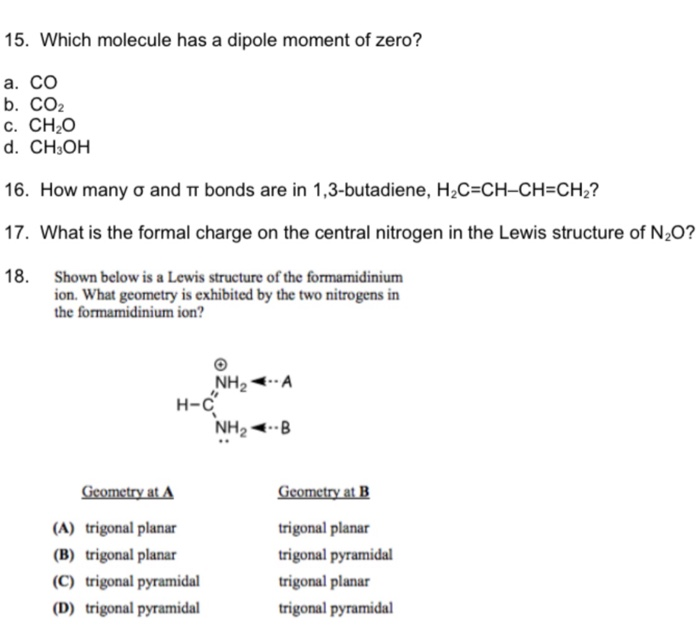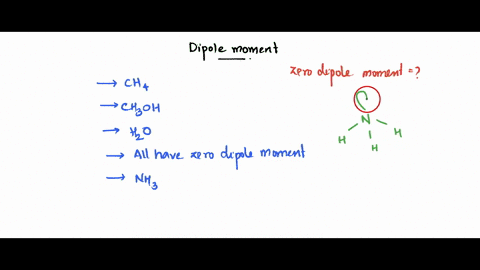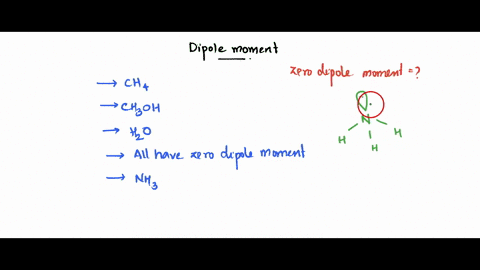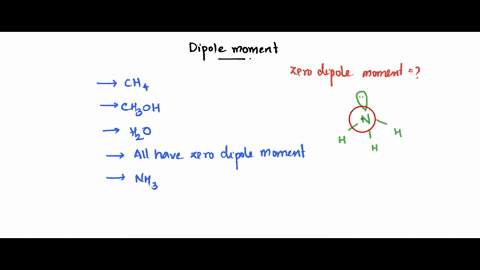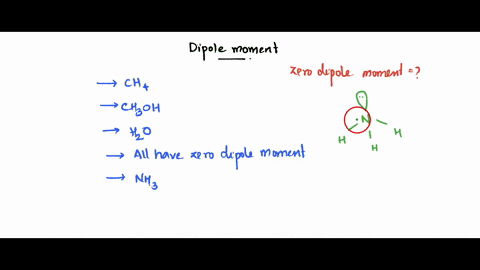
SOLVED: Which of the following molecule has a zero dipole moment? CH4 CH3OH H20 All have zero dipole moments NH3

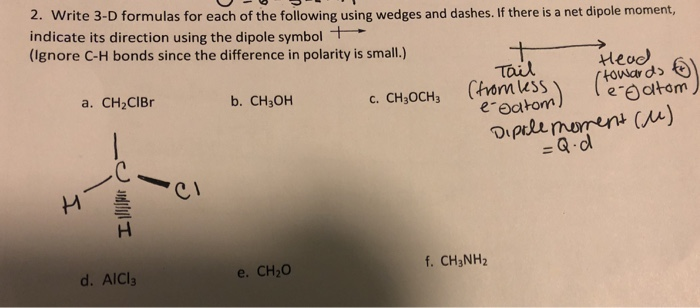
20. Arrange the following in the order given below: a) Decreasing dipole moment : CH3C1, CH2Cl2, CHCI, & CCI. b) Increasing order of dipole moment : CH3OH, CH4, CF., COCHE

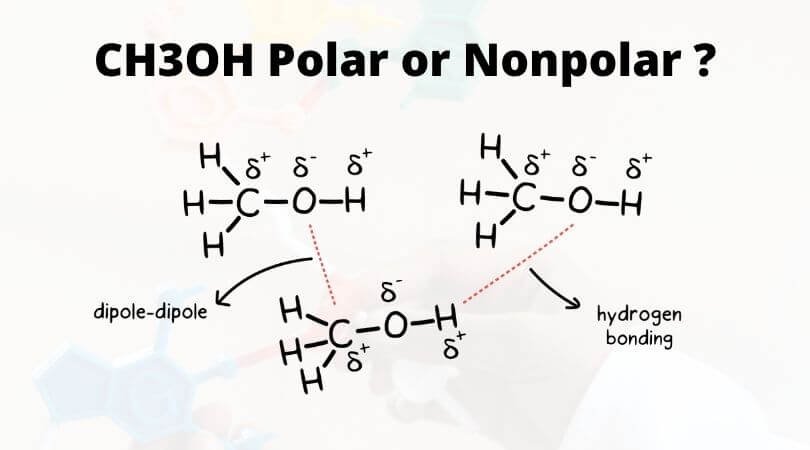
Which drawing best accounts for the polarity of methanol, CH3OH, and the bond polarities that make a major - brainly.com

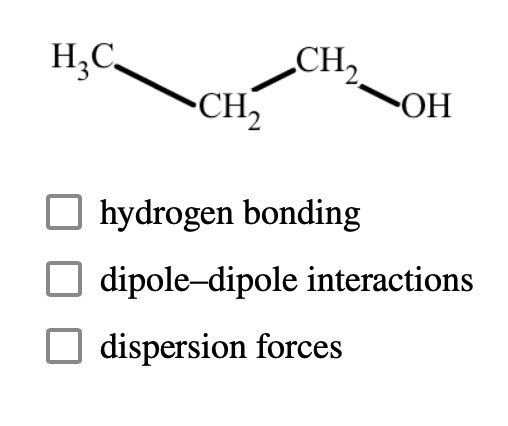
Consider what happens when liquid CCl4 dissolves in liquid CH3OH. (a) What type of attractive forces must be overcome in the liquid CH3OH? (b) What type of forces must be overcome in