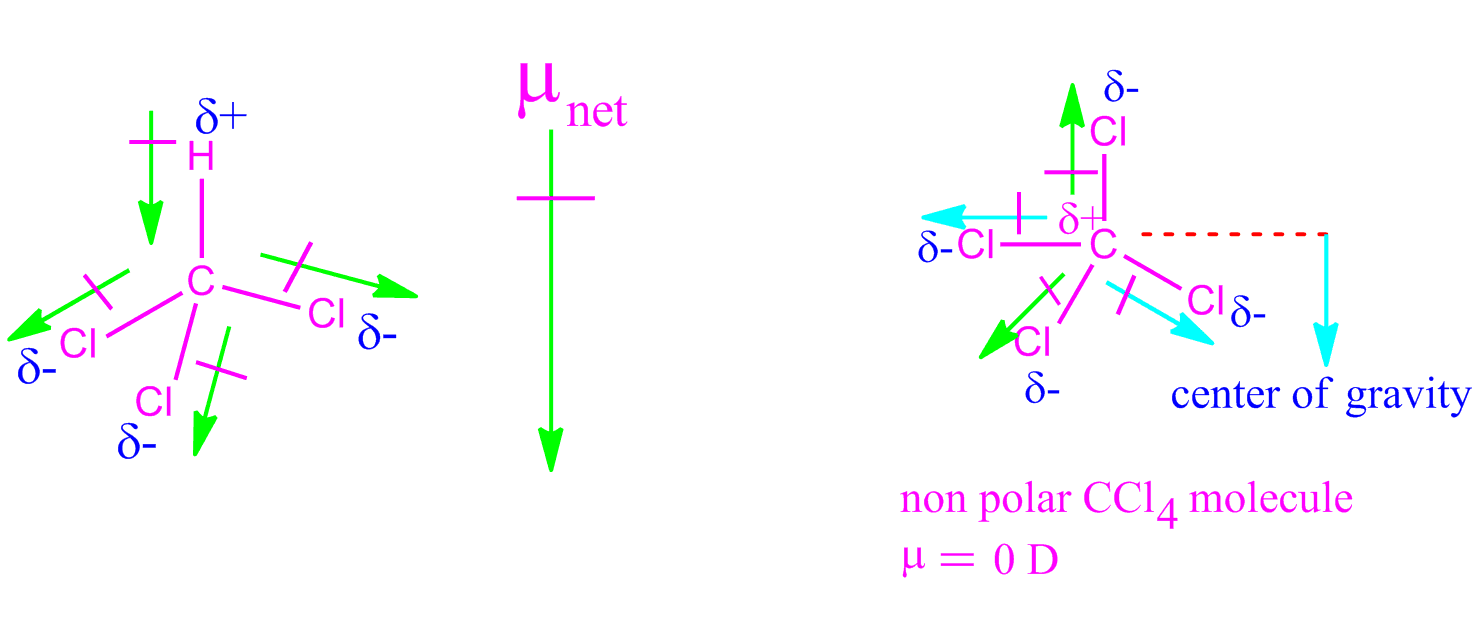Correlation between the Dipole Moment of Nonfullerene Acceptors and the Active Layer Morphology of Green-Solvent-Processed P3HT-Based Organic Solar Cells | ACS Sustainable Chemistry & Engineering

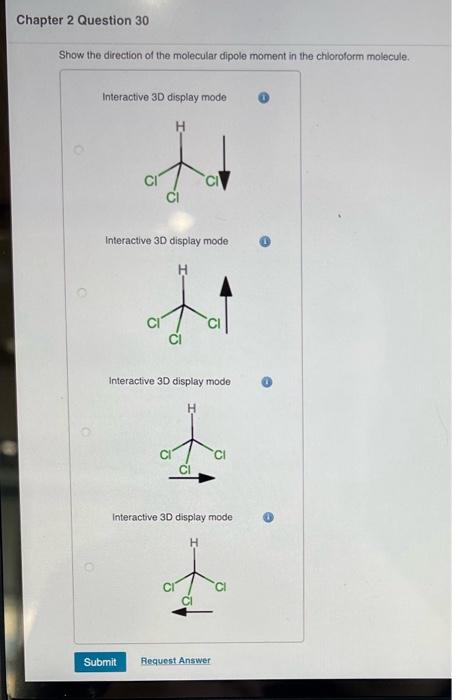
Between chloroform and methyl chloride , whose value of dipole moment is greater and why - Chemistry - - 8779339 | Meritnation.com

halides - Why does chloromethane have a larger dipole moment than chloroform? - Chemistry Stack Exchange

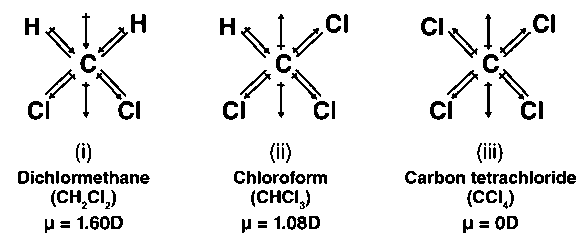
Out of CH2Cl2,CH4,CCl4,H2O,CHCl3,d−dichlorobenzene, o-cresol, p-xylene, SCl2,BF3,IBr and CH2O, non zero value of dipole moment are:
![The dipole moments of \\[CC{{l}_{4}}\\], \\[CHC{{l}_{3}}\\] and \\[C{{H}_{4}}\\]A. \\[C{{H}_{4}}\\]= \\[CC{{l}_{4}}\\]\\[CHC{{l}_{3}}\\]B. \\[CC{{l}_{4}}\\]\\[C{{H}_{4}}\\]\\[CHC{{l}_{3}}\\]C. \\[C{{H}_{4}}\\]\\[CC{{l}_{4}}\\]\\[CHC{{l}_{3}}\\]D ... The dipole moments of \\[CC{{l}_{4}}\\], \\[CHC{{l}_{3}}\\] and \\[C{{H}_{4}}\\]A. \\[C{{H}_{4}}\\]= \\[CC{{l}_{4}}\\]\\[CHC{{l}_{3}}\\]B. \\[CC{{l}_{4}}\\]\\[C{{H}_{4}}\\]\\[CHC{{l}_{3}}\\]C. \\[C{{H}_{4}}\\]\\[CC{{l}_{4}}\\]\\[CHC{{l}_{3}}\\]D ...](https://www.vedantu.com/question-sets/a88bad04-0aa4-49a9-876d-20166fda63e72667075395122850728.png)
The dipole moments of \\[CC{{l}_{4}}\\], \\[CHC{{l}_{3}}\\] and \\[C{{H}_{4}}\\]A. \\[C{{H}_{4}}\\]= \\[CC{{l}_{4}}\\]\\[CHC{{l}_{3}}\\]B. \\[CC{{l}_{4}}\\]\\[C{{H}_{4}}\\]\\[CHC{{l}_{3}}\\]C. \\[C{{H}_{4}}\\]\\[CC{{l}_{4}}\\]\\[CHC{{l}_{3}}\\]D ...