Which of the following would have a permanent dipole moment? (a) SiFa (b) SF4 - Sarthaks eConnect | Largest Online Education Community

Dipole moment of CH4 is zero but it's not the same for SF4 Please answer the 2nd part - Chemistry - Chemical Bonding and Molecular Structure - 12818615 | Meritnation.com
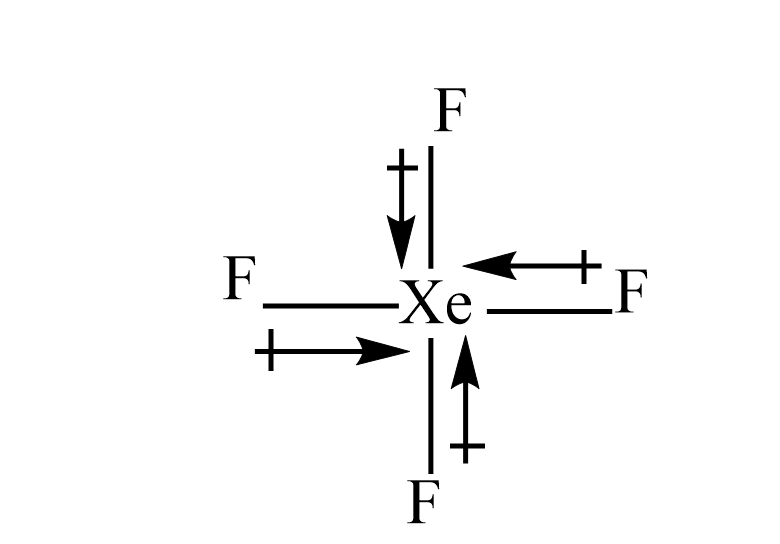
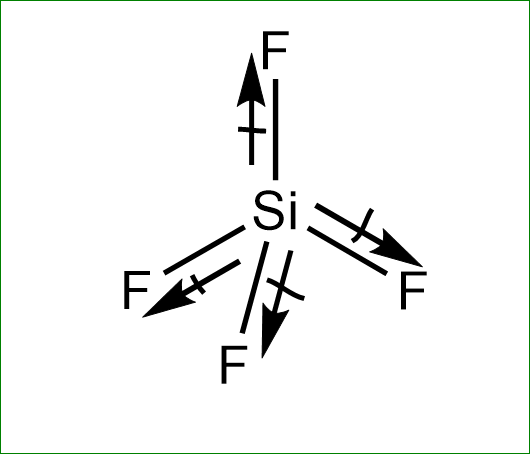
Molecule having a non zero dipole moment?a) $ S{F_4} $ b) $ Si{F_4} $ c) $ Xe{F_4} $ d) $ B{F_3}^{} $
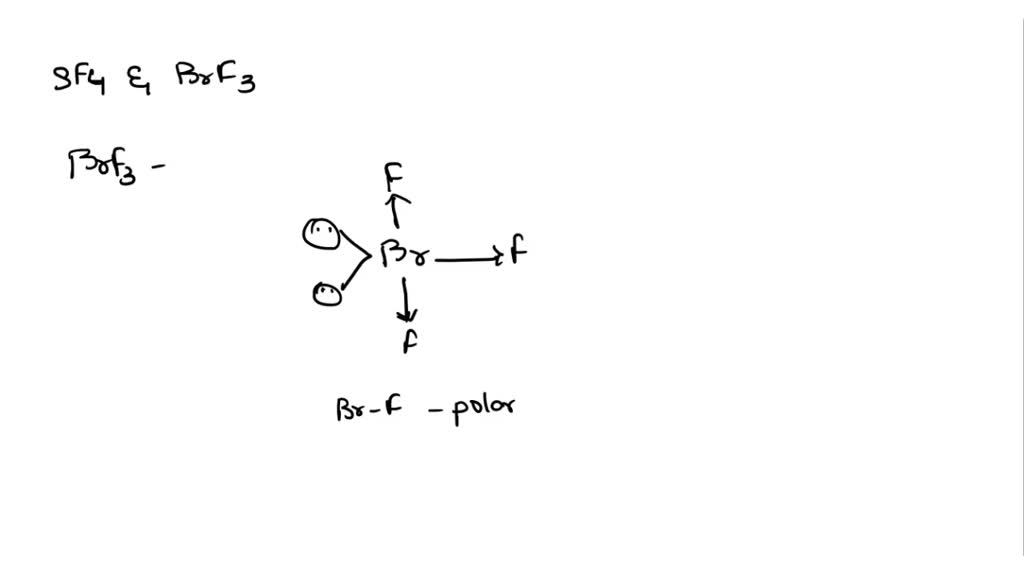
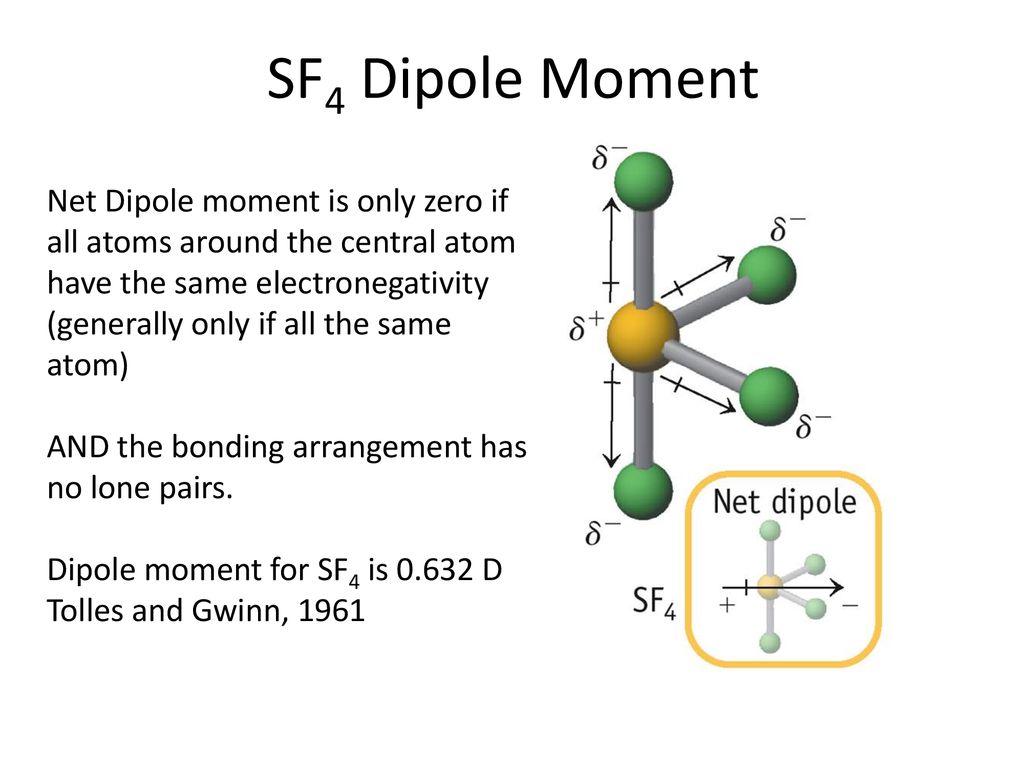
SOLVED: Please explain the dipole moments in SF4 and BrF3. Are they polar? Please draw the direction of the dipole moment with arrows on the preferred structure for each.

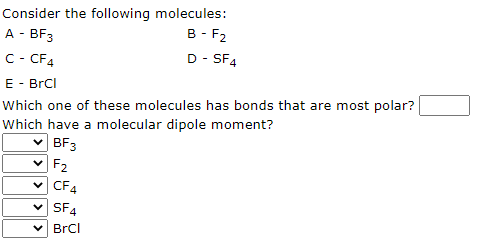
Which have a molecular dipole moment? (Select all that apply.) 1. BF3 2. SF4 3. BrF3 4. NF3 5. CF4 | Homework.Study.com
Welcome to Chem Zipper.com......: Why SF6 is inert while SF4 is a very reactive molecule that reacts with H2O rapidly and vigorously?
✓ Solved: Explain why CF4 and XeF4 are nonpolar compounds (have no net dipole moments) while SF4 is polar...

Who has high dipole moment - SF4 or CF4 pl ans - Chemistry - Coordination Compounds - 13090037 | Meritnation.com